Researchers at UHN's McEwen Stem Cell Institute shed light on the role of cardiac fibroblasts in heart development and disease, key cells for improving treatments for heart-related conditions, specifically those affecting the heart muscle.
Cardiac fibroblasts make up approximately 15–30% of all heart cells and are essential for both fetal heart development and maintenance of the adult heart structure. The majority of the fibroblast population is derived from the outer layer of the heart, called the epicardium.
"There's a lack of laboratory models that accurately replicate the formation and function of the epicardium, which hinders our understanding of heart development, disease mechanisms, and the advancement of new therapies," explains Dr. Gordon Keller, Director of the McEwen Stem Cell Institute and senior author of this study.
To address this gap, the team developed cardiac organoids, three-dimensional pluripotent stem cell-derived structures, designed to mimic key aspects of human heart development and function. These organoids spontaneously form the functional muscle tissue and epicardium found in the heart, providing a platform to study cardiac cell interactions in a more physiologically relevant environment.
Using advanced techniques to monitor cell differentiation and maturation, researchers discovered that interactions between epicardial cells and heart muscle cells (cardiomyocytes) within the organoids resulted in the specification and migration of fibroblasts, in a pattern similar to that observed in developing human hearts.
"Our study demonstrates that this organoid platform serves as an advanced model system for studying multicellular mechanisms underlying heart disease," explains Dr. Ian Fernandes Scientific Associate at McEwen Stem Cell Institute and first author of this study.
Moreover, single-cell RNA sequencing, a cutting-edge technique that allows scientists to study the molecular profile of individual cells, revealed significant diversity within the populations of fibroblasts and cardiomyocytes in the cardiac organoids. This diversity resembled what is seen in both healthy and diseased adult hearts.
Researchers also identified distinct subpopulations of fibroblasts, including a group expressing the CD9 protein, which has been associated with reparative functions.
"The identification of distinct subpopulations of cells within the heart organoids, especially those with reparative properties like the CD9+ fibroblasts, opens up new avenues for understanding and potentially treating heart diseases," adds Dr. Keller. "By targeting these specific cell types, we may develop more precise and effective therapies for various heart-related conditions."
This research opens avenues for understanding cardiac development, disease mechanisms, and potential regenerative therapies, promising advancements in heart-related treatments. Additionally, the ability to isolate and co-transplant specific fibroblast subpopulations with cardiomyocytes in damaged hearts could hold potential for generating new heart muscle and improving heart function.
This work was supported by the Canadian Institutes of Health Research and UHN Foundation. Dr. Gordon Keller is a Professor at the Department of Medical Biophysics at the University of Toronto.
G.M.K. is a scientific co-founder and paid consultant for BlueRock Therapeutics LP and a paid consultant for VistaGen Therapeutics.
Fernandes, I., Funakoshi, S., Hamidzada, H. et al. Modeling cardiac fibroblast heterogeneity from human pluripotent stem cell-derived epicardial cells. Nat Commun 14, 8183 (2023). https://doi.org/10.1038/s41467-023-43312-0
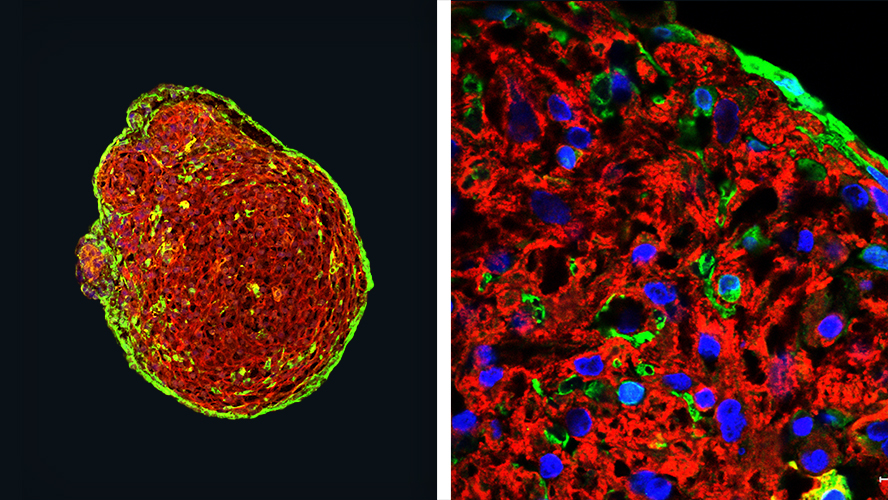
(L) Whole organoid showing cardiomyocytes dyed in red and epicardium in green. (R) Magnified x5 view of a section from the left image.

